What to do with used medical equipment?
According to Doctor of Nursing Practice (DNP) modern medical equipment have become major defenses against illness and disease that in the past would have resulted in the deaths of countless people in the Australia and abroad. In countries like Australia, most individuals have varying degrees of access to the medical equipment that they need.
When they are not used they, like most broken or unneeded equipment, must be disposed of. However, getting rid of one’s medical equipment, particularly items with syringes, isn’t as simple as tossing them in the trash. This equipment must be disposed of carefully and properly, as syringes and other injectable can spread diseases or injure humans and animals or even cause death in those they were not intended for. And improper disposal can also result in pollution of the water supply. With proper education on the right techniques for disposal, the chances of causing an accidental death or injury can be minimized.
About Medical Equipment
What is Medical Equipment?
As defined by the World Health Organization (WHO), medical equipment are medical devices requiring calibration, maintenance, repair, user training, and decommissioning – activities usually managed by clinical engineers. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; it can be used alone or in combination with any accessory, consumable or other piece of medical equipment. Medical Equipment excludes implantable, disposable or single-use medical devices.
What is Used Medical Equipment?
When medical equipment becomes obsolete or unusable or is no longer required by the health care facility, and enters the final stage of its life cycle: decommissioning and disposal.
Stages In Disposing Medical Equipment
Storage of broken and unwanted medical equipment can be dangerous, and can lead to accidental injury, public and environmental harm. As a courtesy to anyone seeking out guidance, we have created recommendations for ways to safely and responsibly dispose your broken or unwanted medical equipment (Figure 1). Using the same principle from WHO’s Decommissioning of Medical Devices, these are three main stages to follow in order to handle the process of used medical equipment disposal from start to finish: Decommission, Decontaminate and Disposal/Reuse
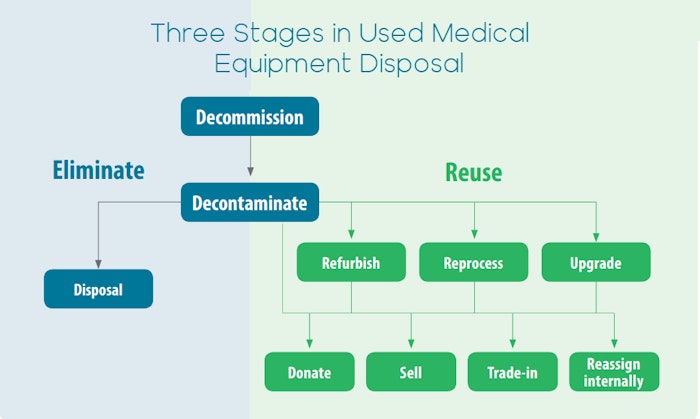
Figure1. From WHO’s Decommissioning of Medical Devices
Decommission: Removal of medical equipment from service in a health care facility after a decision to disinvest in the device itself or in a service in which it is used.
Decontaminate: All used or potentially used medical equipment must be cleaned, as in the instructions specific for the device or type of device.
Disposal or Reuse: Disposal is permanent elimination (e.g. recycling, cannibalizing or incineration) and Re-use (i.e. donated, sold, refurbished, reprocessed, traded-in or reassigned internally to another location)
Decommissioning
The requirement for medical equipment should be appropriately assessed before a decision to decommission is made. If the medical equipment is determined to be obsolete or should be discontinued, it should be:
- Collected and sorted according to treatment and disposal requirements, taken from the source of waste (i.e. the ward, operation theatre, laboratory and bedside) and;
- Categorized into non-hazardous and hazardous equipment waste.


Decontamination
The next stage is to ensure that the equipment is safe for handling and treatment or removal, by cleaning and decontaminating it and removing patient data. The collected equipment is taken to decontamination facilities or a third party professional decontamination organization.
The steps in decontamination depend on the validated instructions provided by the manufacturer and the risk of infection posed by the devices. For example, “Class II” risk equipment should be ideally be thoroughly cleaned and decontaminated and then sterilized, thermally disinfected or pasteurized, while “High Risk Equipment” should be thoroughly decontaminated, cleaned, dried, re-packaged and re-labelled before sterilization.
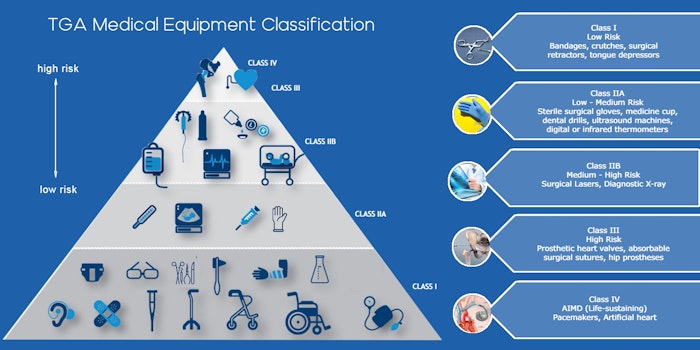
Figure 2. Medical Equipment Classification According to Risk by TGA
The basic steps in cleaning and disinfection that are included in most instructions are as follows.
1. Disassemble. The equipment should generally be disassembled and sorted (Disassembly instruction may be included in the manufacturer’s manual) . Any consumables and sharps must be removed and properly disposed of according to health care waste management procedures.
2. Clean. The equipment should then be cleaned with a compatible cleaning agent such as a detergent (alkaline or pH neutral) or enzymatic solution. It should be submerged in water and rubbed, scrubbed or brushed to remove air pockets and prevent aerosolized transmission of infection. As not all equipment can be submerged in water, it is imperative to follow the instructions. Once the equipment has been cleaned of debris, it should be thoroughly rinsed with detergent and inspected.
3. Manual cleaning. Grossly soiled equipment should be cleaned manually before being processed in a mechanical washer. If an automated washer is unavailable, manual cleaning steps, as outlined in the instructions, should be followed.
4. Mechanical cleaning. Mechanical washer– disinfectors and ultrasonic washers increase the effectiveness of cleaning. They are designed to remove microorganisms by cleaning and rinsing thermally or chemically and/or cavitation.
5. Disinfect. Equipment that are not intended for immediate reuse (e.g. for relocation, trade-in, donation or disposal) should, at a minimum, be thoroughly disinfected thermally or pasteurized after thorough cleaning. Sterilization is recommended and preferred for complete removal of microorganisms.
6. Removal of patient data. Patient data and information stored in medical devices should be erased or removed by the person responsible for the information before a medical device is decommissioned (regardless of its end status) to protect sensitive information and patient confidentiality.
Further information about decontamination can be obtained from a comprehensive WHO guide on decontamination and reprocessing of medical devices for health care facilities.
Disposal or Re-Use
If the medical equipment has been determined to be safe for reuse by the national regulatory authority, the following options below can be chosen:
1. Reassignment. Transfer internally or externally to another unit or facility that requires it. Internal reassignment of a medical device refers to its transfer within the health care system. When the performance of the device is still acceptable and reliable, its relocation to another department that needs it may be considered. It is essential to determine whether the device qualifies for reuse on the basis of a lower acuity application, availability of training, maintenance and other resources. (e.g. Relocating a patient monitor from an emergency department to a general ward )
2. Refurbishing.
Consists of reconditioning used medical devices with no significant change in their performance, safety specifications or service procedures as defined by the manufacturer and its original intended use
3. Donation. Medical equipment can be donated when the safety and performance conditions can be met. For example, a hospital may consider donating medical equipment directly to a low- or middle income country hospital or to a specialized nongovernmental organization, usually as an exchange, which will be responsible for preparing the equipment for re-use and assigning it to a health facility that needs it and that has sufficient capacity and means to use the device wisely.
4. Sale. Decommissioned medical equipment can be transferred within a jurisdiction or sold to another health care facility that needs them, in accordance with the regulations in force.
5. Trade-in. Some medical equipment is procured on a lease or on loan. “Trade-in” refers to returning medical devices to a vendor at a predetermined value towards the purchase of a new device or upgrade.
If the used medical equipment is not qualified for methods above, then it should be identified for disposal. Disposal methods generally involves 2 steps:
1. Treatment. Used Medical equipment waste should undergo thermal, chemical, radiation, biological or mechanical treatment. For hazardous (infectious) waste, disinfection or sterilization is required to minimize potential disease transmission
2. Elimination by:
A. Incineration.Destruction of waste materials via burning, is often used to dispose of used equipment waste when the equipment waste does not have a high metal content. Incineration can be subcategorized into RCRA and non-RCRA incineration, depending on the material incinerated.
B. Landfills. Waste medical equipment that is high metal content is typically sent to landfills if they cannot be recycled.
Special Cases of Medical Equipment Decommissioning
1. Sharps. Any sharp or pointed objects and material that can cause injury and/or infection. It must be handled and disposed safely to reduce the health risks to waste collectors, landfill workers, and the general community. Disposal of this medical equipment requires careful waste management. All sharps used on patients, in isolated wards and from laboratory waste confer a high risk of infection with communicable diseases and are categorized as infectious waste.
All sharps should be stored in a labeled, puncture-proof, leak-tight container, such as FDA-approved sharps containers (e.g. Figure 3). Read further information about managing and disposing sharps.


Safe Disposal of Injectable Products at Home or Work
One doesn’t need to be a nurse to use injectable products. People may use these items at home to pierce or puncture the skin for injections or for testing such as checking one’s blood sugar. FDA approved sharp containers are available at pharmacies, medical facilities, and medical supplies company.
- Write the words “Not recyclable—contains sharps” on the container with a black permanent marker.
- Do not over-fill the container with sharps. This can increase pressure on the lid and cause the sharps to spill out.
- Secure the lid to the container with heavy-duty tape such as electrical or duct tape.
If one is unable to get an FDA-approved container, use a strong container that cannot be pierced by a sharp, made from heavy/thick plastic and with a tight fitting or childproof lid. e.g. a plastic bleach bottle. (Do not use glass or aluminium containers as they are not puncture/shatter resistant).
These should be dropped off at approved collection locations such as pharmacies or hospitals. You can dispose of Australian standard yellow sharps containers at the collection points on the following webpage for example:
- The Pharmacy Guild of Australia
- You can also dispose of home-generated sharps containers in a Sunshine Coast, Noosa or Gympie Council home wheelie bin (not recycle) provided the sharps are in a suitable sealed container described above.
2. Equipment Containing Mercury. Mercury is a heavy metal that evaporates easily into the atmosphere. Inhaled elemental mercury vapour can have harmful effects on the nervous, digestive and immune systems and the lungs and kidneys. Once in the environment, mercury can be transformed by bacteria into methylmercury, which people are exposed to through consumption of contaminated fish and shellfish. Further information can be obtained from the 2015 WHO guidelines on phasing out mercury-containing thermometers and sphygmomanometers in health care.
This equipment should be collected for hazardous waste disposal or for designated recycling. An area dedicated to mercury waste within health care facilities should be determined before the waste is picked up for disposal. The next step is to send mercury waste to authorized facilities or to the original suppliers, if applicable. Another alternative is to send it to a disposal or storage site designated for hazardous industrial waste. For more information, see the WHO guidelines on safe management of wastes from health-care activities .
3. Radioactive Sources. Decommissioning of radioactive sources usually ends with their disposal. The activity of radioactive materials should meet the clearance level before the waste is released from the health facility to be processed further. Until radioactive sources have met the clearance level, they should be stored within health care facilities, with proper handling, in a lead container or in a lead-shielded room to prevent transmission to the environment.
They should be clearly labeled, with information on the types and activity at a given date. WHO recommends lead boxes for storing radioactive waste, labeled with appropriate hazard symbols. Further information about radioactive sources disposal, see the published guidelines in WHOs Disposal options for disused radioactive sources, Management of radioactive waste from the use of radionuclides in medicine, and Transportation of radioactive material.















